The capsule sponge test is a non-endoscopic test for the earlier detection of oesophageal cancer, which is aiming to change the care pathway for people living with chronic reflux or Barrett’s oesophagus. As part of the SBRI Healthcare programme, project CYTOPRIME piloted the real-world implementation of a capsule sponge test, using Medtronic’s Cytosponge™ device, in primary and community care to provide support for the recovery of endoscopy services across the North West Coast.
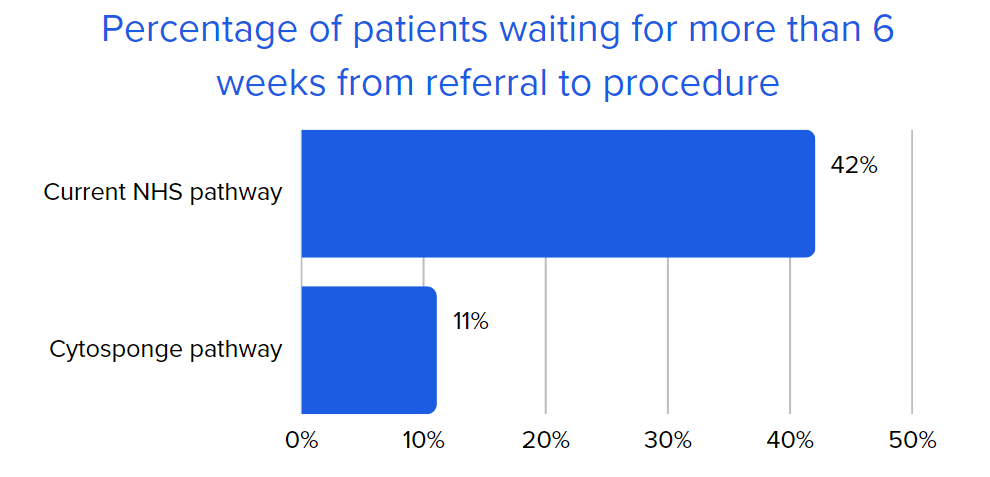
Through offering patients an alternate option to endoscopy that can be delivered in a primary care or community setting, a capsule-sponge test can reduce demand on secondary care resources and reduce waiting times between referral and diagnosis. The goal of the project was to examine and evaluate the delivery of the capsule sponge test in primary and community care settings and the impact of safely managing patients on endoscopy waiting lists.
As independent evaluators of the CYTOPRIME project, Unity Insights:
- Hosted logic model workshops to develop logic models and an evaluation framework.
- Identified suitable public and bespoke data sources for evaluation.
- Performed a quantitative assessment of treatment data received from patients who took part in the pilot, to assess against the existing endoscopy pathway.
- Performed a qualitative assessment of patient and staff feedback regarding the implementation of the capsule sponge test.
- Constructed a return-on-investment model to assess the impact of the capsule sponge test implementation across different scenarios.
- Developed a bespoke budget impact model to allow stakeholders to assess the expected changes in expenditure, as a result of implementing the capsule sponge test.
The findings at the conclusion of the pilot offered valuable insights into the implementation of the capsule sponge test within primary and community care. The results suggest that the implementation and operating costs of the capsule sponge test are outweighed by the benefits it contributes to patients, staff, and the wider health and social care system.
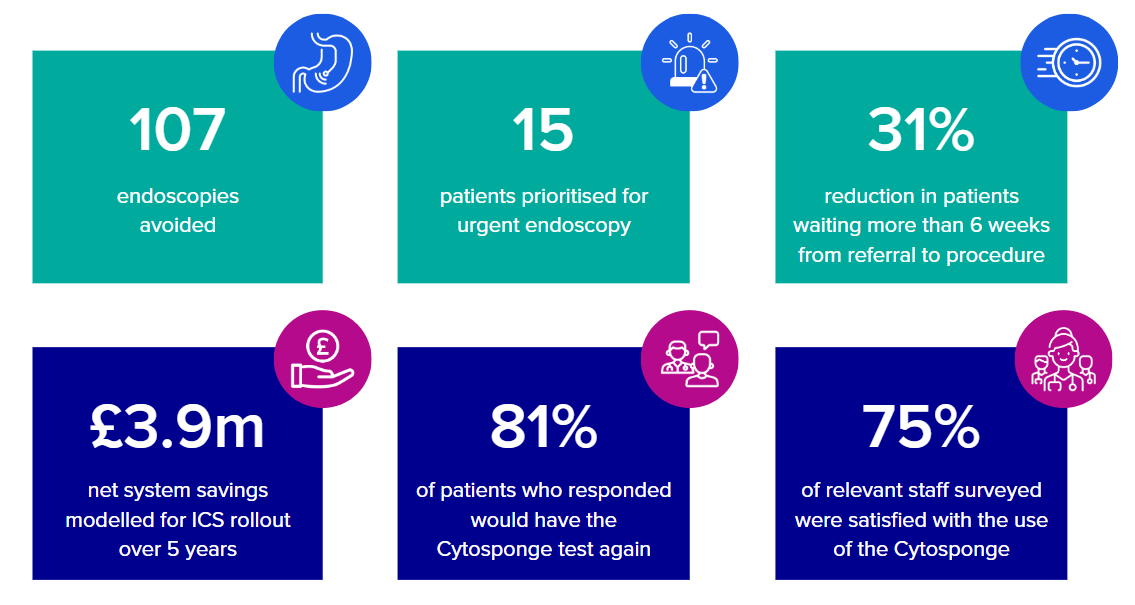
A positive return on investment was seen when modelling implementation and outcomes of the project over a 12-month period, with a further increase seen over a period of 5 years.

Patients were happy to have an alternative option to an endoscopy. Feedback varied but was positive in noting uptake factors such as shorter waiting times, the speed or ease of procedure, the less invasive nature of the test, and the convenience of attending the appointment alone and returning to daily activities.
Staff praised the collaboration across teams and opportunities for upskilling, and the impact on the system in clearing the Barrett’s surveillance endoscopy backlog at four trusts. The stakeholders’ efforts in addressing staff and patient feedback to improve the future implementation and delivery of capsule sponge tests is promising for future spread and adoption.
“The findings of this report clearly demonstrate the huge positive impact of capsule sponge technology. They show that we can cut waiting times, deliver earlier diagnoses, and help to relieve pressure on health systems still recovering from the pandemic. By making this technology widely available in communities, we can diagnose cancer earlier, improving patient outcomes.”
Cyted
Another grant has since been awarded for a second project as part of the NHS Cancer Programme, supported by SBRI Healthcare and Accelerated Access Collaborative. CYTOPRIME2 will offer capsule sponge tests using Cyted’s EndoSign® across primary and community care settings for patients living with Barrett’s oesophagus or chronic reflux. Unity Insights are excited to continue to explore the impact of capsule sponge tests for patients, staff, and the wider healthcare system.
Device information: The capsule sponge test was developed by a team at the Medical Research Council (now University of Cambridge). The Cytosponge™ devices used in the CYTOPRIME project were manufactured by Medtronic, with Cyted Ltd providing the diagnostic analysis and clinical reporting.

This work was commissioned and funded by the SBRI Healthcare programme. SBRI Healthcare is an NHS England initiative, championed by the Academic Health Science Networks (AHSNs). The views expressed in the publication are those of the author(s) and not necessarily those of the SBRI Healthcare programme or its stakeholders. Full methodologies, limitations, and considerations can be found in the full evaluation report.
Download additional information
Related content
Get in touch with us
- Phone
- Email enquiries@unityinsights.co.uk
